How do dissolved oxygen sensors work? Technology makes it quick and easy
Dissolved oxygen sensors are one of most important and useful tools in the toolbox of an aquaculture or fisheries manager. Regular, rapid and reliable reporting of dissolved oxygen (DO) levels in an enclosure can mean the difference between a healthy, profitable fish stock and sick or dying animals.
While reliable methods of determining oxygen levels have existed for decades, advances in technology continue to improve accuracy and ease of use. Today’s sensors can be left in the field longer, send updates wirelessly, and provide more accurate readings.
The most common types of DO sensors fall into two categories: electrochemical and optical. They’re explained below, but if you feel you need more information, visit the Fondriest Environmental Learning Center.
Electrochemical sensors
Electrochemical sensors, as their names suggest, rely on the flow of electricity and chemical reactions within the sensor.
The two main types of electrochemical sensors, galvanic and polarographic sensors, work in similar ways. However, key differences in their functioning influence the user’s experience.
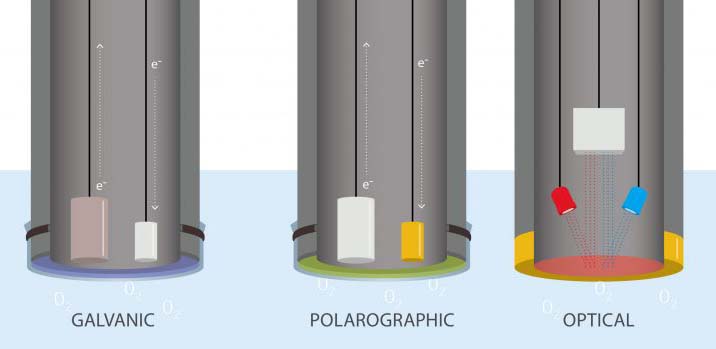
In each type of sensor, two electrodes are surrounded by a semipermeable membrane. That membrane lets dissolved oxygen through, up to the concentration of dissolved oxygen in the surrounding water. Within the confines of the membrane, it dissolves into an electrolyte solution of, for example, sodium chloride (NaCl) or potassium chloride (KCl). At this point, the sensors differ significantly.
In a galvanic sensor, the two electrodes, the cathode and anode, either give an electron to the oxygen or receive one from it. This exchange creates an electrical current. The sensor measures the strength of this current, which can be converted into a dissolved oxygen measurement: the stronger the current, the more oxygen available.
In a polarographic sensor, the electrical current isn’t generated by the electrodes. Instead it’s supplied to them by an external source of electricity, like a battery pack. Here, the two electrodes are still suspended in an electrolyte solution within a semipermeable membrane, which allows dissolved oxygen through. Now, instead of measuring the current, the sensor measures the changes to the current caused by the presence of oxygen. Because the reaction isn’t spontaneous, there is a brief warm-up period for this type of sensor, usually five to ten minutes.
In order to get accurate readings, both types of electrochemical sensors need to be in water with at least some movement. The reactions that make the sensor work consume the dissolved oxygen. Without new water flowing over the sensor and new dissolved oxygen permeating through the membrane, readings will drop below actual levels as the oxygen is used up.
Optical Sensors
Optical dissolved oxygen sensors work entirely differently from their electrochemical counterparts. Instead of measuring an electrical current running through a solution, they measure light.
Optical sensors still have a semipermeable membrane that allows oxygen through. When blue light shines through the solution, it interacts with a luminescent dye that glows when it interacts with blue light. In some optical sensors, those that are luminescence intensity-based, dissolved oxygen is measured by the amount of light that reaches the detectors. Because oxygen decreases the amount of light that reaches the sensor, the more light it receives, the less oxygen present. In another type of optical sensor, which measures the lifetime of the luminescence, the longer the light lasts, the less dissolved oxygen present.
A dissolved oxygen sensor for every need. Credit: Fondriest Environmental
Other manual methods
Sensors like the ones described above are replacing older, manual methods of taking dissolved oxygen measurements.
Titration, in the simplest terms, is adding one solution with a known concentration (called a titrant) to another until an indicator reaction occurs. The most widely used titration method for determining dissolved oxygen is called the Winkler method. In it, dissolved oxygen is determined by measuring the amount of titrant needed to force a color change.
Titration is a multi-step process that requires careful measurements and calibration of instruments throughout the process. With care, it can be a precise, if slightly unwieldy, method of measurement, though modern handheld sensors are much quicker and reliable.
The other manual method, colorimetry, involves adding a color-changing substance to a water sample. The intensity of the resulting color is then judged against a key to determine the dissolved oxygen levels. Two of the more common methods involve indigo carmine and rhodazine D. Based on levels of dissolved oxygen present, indigo carmine turns water blue at differing intensities while rhodazine D turns water rosy pink. Both tests can be influenced by other water characteristics and can be time-sensitive at different dissolved oxygen levels. These tests rely, at least in part, on a judgment call.
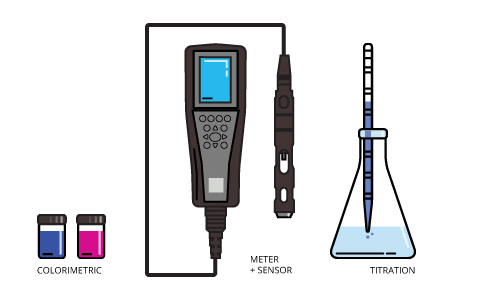
A proven dissolved oxygen measurement technique is available for any aquaculture need. Credit: Fondriest Environmental
Choosing the right dissolved oxygen sensor
While the three categories of most widely used dissolved oxygen can all provide quick and accurate measurements, they’re not equal in all ways.
Galvanic sensors, those that spontaneously generate an electrical current, will need repair from time to time as the electrodes break down because of the constant oxidation and reduction reactions.
Polarographic sensors, on the other hand, will require less repair over time, since the oxidation and reduction reactions only occur when an electrical current is provided and because the electrodes are made of less reactive metals, they tend to last longer. Both electrochemical sensors, though, typically employ thin membranes. This means they require fairly regular maintenance and calibration.
Between the two electrochemical sensors, galvanic sensors are quicker to respond. As was mentioned above, taking a measurement with a polarographic sensor requires a brief warm up period of a few minutes.
Optical sensors are the newer, longer lasting but more expensive option. The greatest advantage is that an optical sensor can be left in the field for up to months because they rely on thicker membranes and don’t have electrodes that can wear down. The main reason they might need maintenance is biofouling or the interference of algae growth. Fortunately, simple wipers can be added to sensors to clear away interfering algae, extending the length of the sensor’s deployment.
Optical sensors are more expensive than electrochemical ones, but require less maintenance and hands-on attention.
How dissolved oxygen sensors changed the game
Dissolved oxygen measurements have been called the most important measurement when it comes to fisheries and aquaculture management.
With easy to use, accurate sensors widely available, it is easier than ever to get dissolved oxygen readings, gather key information about ecosystem health or keep fish and shellfish from feeling the ill-effects of low dissolved oxygen.
What used to require careful execution of a long process or imprecise judgment calls, can now be had by simply sticking a sensor in the water. With wireless technology, sensors can beam measurements back from the field, eliminating frequent trips.
Dissolved oxygen sensors aren’t brand new, by any means. But the steady march of innovation, from titration to colorimetry to electrochemical sensors to optical ones, means there’s already a method of dissolved oxygen measurement that fits your needs.
Remember that if you’re still hungry for more information, visit the Fondriest Environmental Learning Center.



0 comments